
Chemistry, 02.10.2020 16:01 ashvinmsingh
calculate the heat required to melt 25.7g of solid methanol (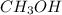 at its melting point ( Hfus=3.22KJ/mol , Mm=32g/mol)
at its melting point ( Hfus=3.22KJ/mol , Mm=32g/mol)


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
calculate the heat required to melt 25.7g of solid methanol ( at its melting point ( Hfus=3.22KJ/mol...
Questions

Mathematics, 09.07.2019 12:30



Mathematics, 09.07.2019 12:30


Biology, 09.07.2019 12:30

Biology, 09.07.2019 12:30






History, 09.07.2019 12:30



Mathematics, 09.07.2019 12:30


Spanish, 09.07.2019 12:30




