
Chemistry, 02.10.2020 14:01 haleyehewitt2001
Attached photo - Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems.
A. –1.68 V
B. –0.78 V
C. 0.78 V
D. 1.68 V
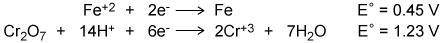

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

You know the right answer?
Attached photo - Using the two cell reduction potentials shown for their corresponding reaction, cal...
Questions

Geography, 03.07.2019 23:00


History, 03.07.2019 23:00


Mathematics, 03.07.2019 23:00




History, 03.07.2019 23:00


English, 03.07.2019 23:00




Biology, 03.07.2019 23:00


Biology, 03.07.2019 23:00






