
Chemistry, 29.09.2020 14:01 andreyvaught2754
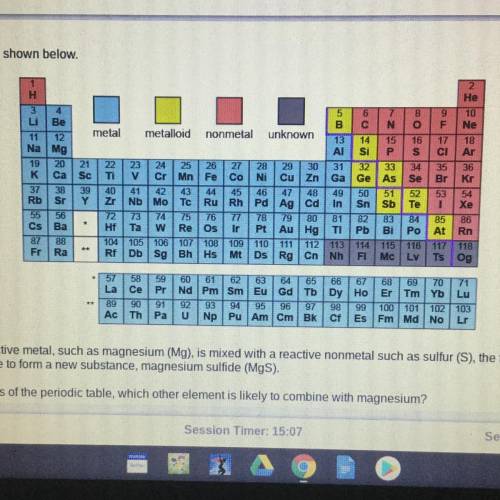
When a highly reactive metal, such as magnesium (Mg), is mixed with a reactive nonmetal such as sulfur (S), the two elements will
most likely combine to form a new substance, magnesium sulfide (MgS).
Based on the trends of the periodic table, which other element is likely to combine with magnesium?
A. Sodium (Na)
B. Helium (He)
C. Oxygen (O)
D. Gold (Au)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
When a highly reactive metal, such as magnesium (Mg), is mixed with a reactive nonmetal such as sulf...
Questions





Mathematics, 28.01.2020 07:31

Physics, 28.01.2020 07:31


Social Studies, 28.01.2020 07:31


History, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

English, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31



Mathematics, 28.01.2020 07:31



History, 28.01.2020 07:31




