
Chemistry, 25.09.2020 09:01 rachel63892
LOL please help me ✨
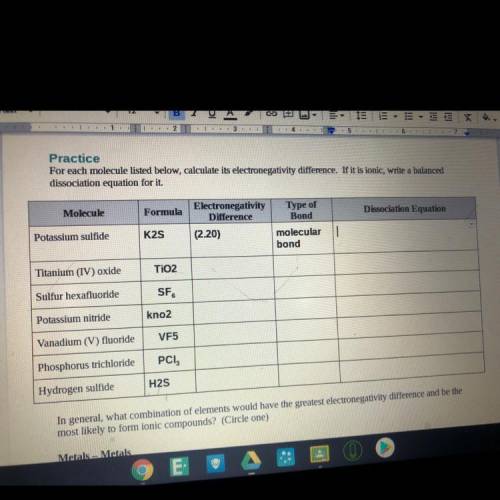
For each molecule listed below, calculate its electronegativity difference. If it is ionic, write a balanced
dissociation equation for it
Dissociation Equation
Molecule
Formula
Electronegativity
Difference
(2.20)
Type of
Bond
molecular
bond
Potassium sulfide
K2S
Titanium (IV) oxide
TiO2
1
Sulfur hexafluoride
SF
Potassium nitride
kno2
Vanadium (V) fluoride
VF5
Phosphorus trichloride
PCI,
Hydrogen sulfide
H2S

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
LOL please help me ✨
For each molecule listed below, calculate its electronegativity difference. If...
Questions



Arts, 10.12.2020 16:20



History, 10.12.2020 16:20



Mathematics, 10.12.2020 16:20





Mathematics, 10.12.2020 16:20



History, 10.12.2020 16:20



History, 10.12.2020 16:20



