
Chemistry, 24.09.2020 15:01 yungking1329
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of virial expansion at (a) its critical temperature and (b) its Boyle temperature. Assume that the pressure is 10 atm throughout. At what temperature is the gas most perfect? Use the following data: Tc = 126.3 K, a=1.352 L2 atm mol-2, b = 0.0387 L mol-1

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
Calculate the molar volume occupied by 1 mole of N2 using the van der Waals equation in the form of...
Questions



Mathematics, 26.09.2019 03:30

Computers and Technology, 26.09.2019 03:30


SAT, 26.09.2019 03:30

Chemistry, 26.09.2019 03:30

History, 26.09.2019 03:30




Mathematics, 26.09.2019 03:30




History, 26.09.2019 03:40


Chemistry, 26.09.2019 03:40


 = 1 mole
= 1 mole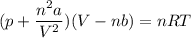

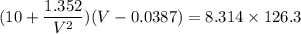

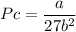
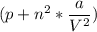 depending on the circumstances.
depending on the circumstances.


