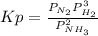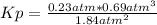Chemistry, 24.09.2020 07:01 20jessicacabriales
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a flask with of ammonia gas, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

Social Studies, 01.04.2020 02:51

English, 01.04.2020 02:51

History, 01.04.2020 02:51


Mathematics, 01.04.2020 02:51



Mathematics, 01.04.2020 02:52

History, 01.04.2020 02:52

English, 01.04.2020 02:52

Chemistry, 01.04.2020 02:52


Mathematics, 01.04.2020 02:52

Mathematics, 01.04.2020 02:52


Mathematics, 01.04.2020 02:52



History, 01.04.2020 02:52

Health, 01.04.2020 02:52