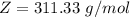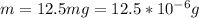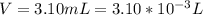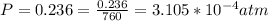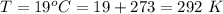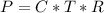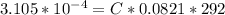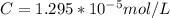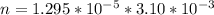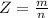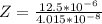Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (12.5 ×10–6 g) of what you hope is pure saxitoxin (a non-electrolyte) from a poisonous (and quite cross) puffer fish. You dissolve this sample in 3.10 mL of water and determine that the osmotic pressure of the resulting solution is 0.236 torr at 19ºC (760 torr = 1.00 atm). What is the molar mass of the compound?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Working on-board a research vessel somewhere at sea, you have (carefully) isolated 12.5 micrograms (...
Questions

Computers and Technology, 27.11.2021 14:00



Mathematics, 27.11.2021 14:00

Biology, 27.11.2021 14:00

English, 27.11.2021 14:00

Chemistry, 27.11.2021 14:00

Mathematics, 27.11.2021 14:00

History, 27.11.2021 14:00



Physics, 27.11.2021 14:00

Physics, 27.11.2021 14:00

Mathematics, 27.11.2021 14:00

Mathematics, 27.11.2021 14:00

Computers and Technology, 27.11.2021 14:00

History, 27.11.2021 14:00

Mathematics, 27.11.2021 14:00


Social Studies, 27.11.2021 14:00