
Chemistry, 22.09.2020 20:01 leannamat2106
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl deposit. Sample #1 is from the top of the well, and is initially at 32°C. Sample #2 is from a depth of 50.m, and is initially at 42°C. Both samples are allowed to come to room temperature (20.°C) and 1atm pressure. An NaCl precipitate is seen to form in Sample #1.
a. A bigger mass of NaCl precipitate will form in Sample
b. A smaller mass of NaCl precipitate will form in Sample
c. The same mass of NaCl precipitate will form in Sample #2.
d. No, precipitate will form in Sample #2.
e. I need more information to predict whether and how much precipitate will form in Sample #2.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl depo...
Questions

History, 04.02.2022 01:00

History, 04.02.2022 01:00


English, 04.02.2022 01:00





English, 04.02.2022 01:00



Mathematics, 04.02.2022 01:00

Mathematics, 04.02.2022 01:00




Mathematics, 04.02.2022 01:00

Chemistry, 04.02.2022 01:00


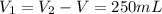
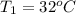
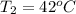
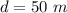
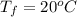
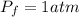
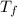 there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one
there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one 

