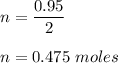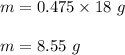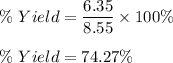Chemistry, 22.09.2020 16:01 shezelleramadoo3451
If 30.2 g of CaO is added to 34.5 g of HCl and 6.35 g of water is formed, what is the percent yield?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
If 30.2 g of CaO is added to 34.5 g of HCl and 6.35 g of water is formed, what
is the percent yield...
Questions





Advanced Placement (AP), 14.01.2021 18:10

Mathematics, 14.01.2021 18:10



Mathematics, 14.01.2021 18:10




Mathematics, 14.01.2021 18:10

Health, 14.01.2021 18:10


Health, 14.01.2021 18:10

Mathematics, 14.01.2021 18:10

English, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10


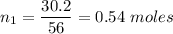
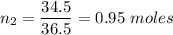
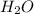 .
.