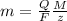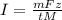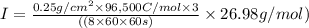The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after an 8 h immersion period. What is the corresponding corrosion current density in mA/em2, assuming that all the corrosion is due to the reaction:
Al → Al3+ + 3e
The atomic weight of Al is 26.98 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
The weight loss of an aluminum (Al) alloy corroding in HCI acid was observed to be 0.250 g/cm2 after...
Questions

Mathematics, 03.05.2021 18:00



Mathematics, 03.05.2021 18:00


History, 03.05.2021 18:00

Mathematics, 03.05.2021 18:00

Mathematics, 03.05.2021 18:00





Mathematics, 03.05.2021 18:00

Mathematics, 03.05.2021 18:00






Mathematics, 03.05.2021 18:00