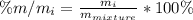Chemistry, 20.09.2020 18:01 kaylaelaine18
A student was provided with a solid sample made up of a mixture of components. Prior to separation, the student measured 2.895 g of the mixture. After separation, the student found the mixture contained the following four components:
Component 1: 1.12g
Component 2: 0.756g
Component 3: 0.254g
Component 4: 0.525g
Which component has the highest mass % for the mixture?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
A student was provided with a solid sample made up of a mixture of components. Prior to separation,...
Questions



History, 01.04.2021 02:10


Mathematics, 01.04.2021 02:10

Mathematics, 01.04.2021 02:10


Mathematics, 01.04.2021 02:10

Mathematics, 01.04.2021 02:10

Mathematics, 01.04.2021 02:10


Mathematics, 01.04.2021 02:10


Chemistry, 01.04.2021 02:10


Mathematics, 01.04.2021 02:10


Chemistry, 01.04.2021 02:10


Mathematics, 01.04.2021 02:10