
Chemistry, 20.09.2020 16:01 aaronscott6474
What is the predominant intermolecular force in the liquid state of each of these compounds: hydrogen fluoride (HF), carbon tetrabromide (CBr4), and methyl chloride (CH3Cl)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
What is the predominant intermolecular force in the liquid state of each of these compounds: hydroge...
Questions

History, 25.06.2019 12:00

Biology, 25.06.2019 12:00



Computers and Technology, 25.06.2019 12:00



English, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00

Biology, 25.06.2019 12:00


History, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00


Mathematics, 25.06.2019 12:00





Social Studies, 25.06.2019 12:00

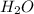 , hydrogen fluoride HF etc
, hydrogen fluoride HF etc 

