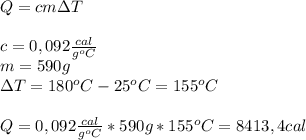Chemistry, 17.10.2019 17:50 21hendlill
The specific heat of copper is 0.092 cal/g °c. if a 590 g copper pan is heated from a temperature of 25 °c to 180 °c, how many calories of heat energy will the copper pan absorb?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

You know the right answer?
The specific heat of copper is 0.092 cal/g °c. if a 590 g copper pan is heated from a temperature of...
Questions

Social Studies, 23.07.2019 10:50



History, 23.07.2019 10:50


Arts, 23.07.2019 10:50

History, 23.07.2019 10:50



History, 23.07.2019 10:50


Biology, 23.07.2019 10:50

Biology, 23.07.2019 10:50




Computers and Technology, 23.07.2019 10:50

Chemistry, 23.07.2019 10:50