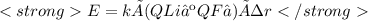Chemistry, 20.09.2020 15:01 briseidam6683
Which factors will result in a stronger ionic bond overall?a. larger ions b. similarity of ionic sizes c. greater absolute charges d. smaller ions

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Which factors will result in a stronger ionic bond overall?a. larger ions b. similarity of ionic siz...
Questions


Mathematics, 31.08.2021 01:20


World Languages, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20



Mathematics, 31.08.2021 01:30

Mathematics, 31.08.2021 01:30


English, 31.08.2021 01:30

Arts, 31.08.2021 01:30



History, 31.08.2021 01:30

Mathematics, 31.08.2021 01:30



Mathematics, 31.08.2021 01:30