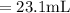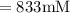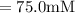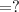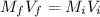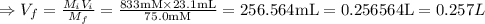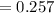A chemist must dilute of aqueous aluminum chloride solution until the concentration falls to . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
A chemist must dilute of aqueous aluminum chloride solution until the concentration falls to . He'll...
Questions

Mathematics, 22.07.2021 06:20

Business, 22.07.2021 06:20

Advanced Placement (AP), 22.07.2021 06:20

Mathematics, 22.07.2021 06:20

History, 22.07.2021 06:20

Mathematics, 22.07.2021 06:20


Mathematics, 22.07.2021 06:20

Mathematics, 22.07.2021 06:20

Advanced Placement (AP), 22.07.2021 06:20

Mathematics, 22.07.2021 06:30

History, 22.07.2021 06:30



Engineering, 22.07.2021 06:30

Mathematics, 22.07.2021 06:30

Mathematics, 22.07.2021 06:30