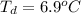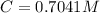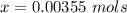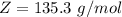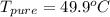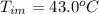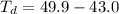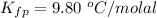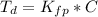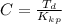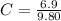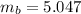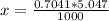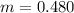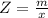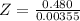Chemistry, 20.09.2020 09:01 davidleew24
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass= 5.047 g Unknown mass= .480 g 1. From the difference between the freezing points of the pure benzophenone and the unknown + benzophenone solution, calculate the freezing point depression of the solution. 2. Given the freezing point depression constant for benzophenone, Kfp= 9.80 C/molal, calculate the molality of the solution of unknown in benzophenone. (answer in m) 3. Now use the calculated value for the molality of the solution and the mass of the benzophenone to compute the number of moles of solute present in the solution. 4. Use that calculated number of moles and mass of solute to determine the approximate molar mass (Gram molecular weight) of the unknown solute. (answer in g/mol)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1

Chemistry, 23.06.2019 12:30
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3
You know the right answer?
Benzophenone freezing point= 49.9 C Benzophenone + unknown freezing point= 43.0 C Benzophenone mass=...
Questions

Biology, 28.07.2019 00:00

Mathematics, 28.07.2019 00:00


Physics, 28.07.2019 00:00


Business, 28.07.2019 00:00

History, 28.07.2019 00:00



Biology, 28.07.2019 00:00

Business, 28.07.2019 00:00

World Languages, 28.07.2019 00:00

Business, 28.07.2019 00:00





Mathematics, 28.07.2019 00:00


Physics, 28.07.2019 00:00