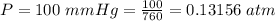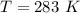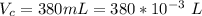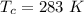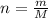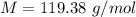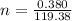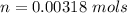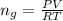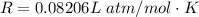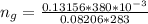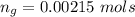Chemistry, 20.09.2020 18:01 juanitarodrigue
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid CHCl3 is placed in a closed, evacuated 380. mL container at a temperature of 283 K.
Assuming that the temperature remains constant, will all of the liquid evaporate? yes/no
What will the pressure in the container be when equilibrium is reached? mm Hg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid C...
Questions

Physics, 25.02.2021 01:00







History, 25.02.2021 01:00






History, 25.02.2021 01:00



English, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00

Mathematics, 25.02.2021 01:00