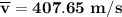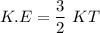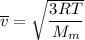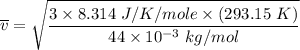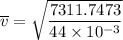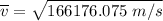Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C. The gases are in thermal equilibrium with one another. a) Which has the higher kinetic energy, the average CO2 or N2 molecule? b) What is that root-mean-square velocity of a CO2 molecule? For reference, a carbon atom has 6 protons, a nitrogen atom has 7 protons, and an oxygen atom has 8 protons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

You know the right answer?
Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C....
Questions

Mathematics, 19.04.2021 18:50






Mathematics, 19.04.2021 18:50

Geography, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50

Mathematics, 19.04.2021 18:50



Mathematics, 19.04.2021 18:50


Arts, 19.04.2021 18:50

Health, 19.04.2021 18:50

Health, 19.04.2021 18:50

World Languages, 19.04.2021 18:50