Chemistry, 20.09.2020 14:01 Serenitybella
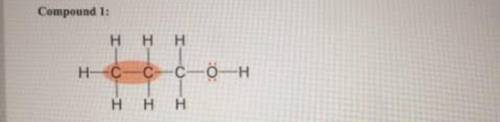
Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond is not a pi bond, select the blank option from the dropdown menu.) Compound 1: Molecular orbital type: Atomic orbitals in the sigma bond: Atomic orbitals in the pi bond: Compound 2: Molecular orbital type: Atomic orbitals in the sigma bond: Atomic orbitals in the pi bond:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond...
Questions














Computers and Technology, 25.12.2019 02:31