
Chemistry, 20.09.2020 08:01 swelch2010
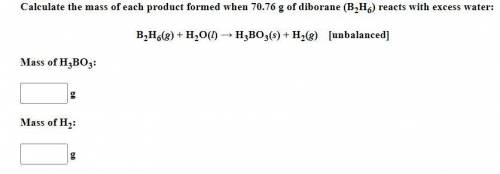
Be sure to answer all parts. Calculate the mass of each product formed when 70.76 g of diborane (B2H6) reacts with excess water: B2H6(g) + H2O(l) → H3BO3(s) + H2(g) [unbalanced] Mass of H3BO3: g Mass of H2: g

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Be sure to answer all parts. Calculate the mass of each product formed when 70.76 g of diborane (B2H...
Questions

English, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50


Mathematics, 19.08.2021 04:50

Biology, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50

Spanish, 19.08.2021 04:50



English, 19.08.2021 04:50

History, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50

Geography, 19.08.2021 04:50

Mathematics, 19.08.2021 04:50







