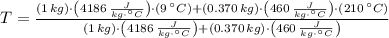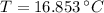Chemistry, 19.09.2020 01:01 jesussanchez1445
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0.370 kg at 210°C into it. After the sizzling subsides, what is the final equilibrium temperature (in °C)? (Make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don't affect the outcome.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

You know the right answer?
A 1.00 kg sample of water at 9.00°C is in a calorimeter. You drop a piece of steel with a mass of 0....
Questions


Mathematics, 31.08.2019 21:30

Arts, 31.08.2019 21:30

Health, 31.08.2019 21:30


Mathematics, 31.08.2019 21:30

History, 31.08.2019 21:30

Mathematics, 31.08.2019 21:30


Chemistry, 31.08.2019 21:30


Computers and Technology, 31.08.2019 21:30

Business, 31.08.2019 21:30


History, 31.08.2019 21:30

Geography, 31.08.2019 21:30

English, 31.08.2019 21:30

History, 31.08.2019 21:30


Business, 31.08.2019 21:30

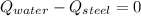
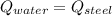
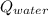 - Heat received by the water sample, measured in joules.
- Heat received by the water sample, measured in joules.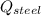 - Heat released by the piece of steel, measured in joules.
- Heat released by the piece of steel, measured in joules.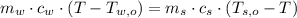
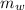 ,
, 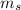 - Masses of the water sample and the piece of steel, measured in kilograms.
- Masses of the water sample and the piece of steel, measured in kilograms.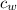 ,
, 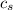 - Specific heat of water and steel, measured in joules per kilogram-Celsius.
- Specific heat of water and steel, measured in joules per kilogram-Celsius.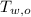 ,
, 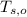 - Initial temperatures of the water sample and the piece of steel, measured in Celsius.
- Initial temperatures of the water sample and the piece of steel, measured in Celsius.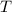 - Final temperature of the sample-piece system, measured in Celsius.
- Final temperature of the sample-piece system, measured in Celsius.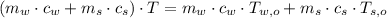
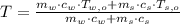
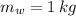 ,
, 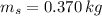 ,
, 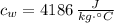 ,
, 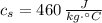 ,
, 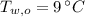 and
and 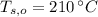 , the final temperature of the system is:
, the final temperature of the system is: