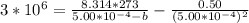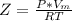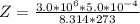Chemistry, 10.09.2020 04:01 llnapier8924
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found to be 5.00 × 10–4 m3 mol−1 at 273 K and 3.0 MPa. From this information calculate the van der Waals constant b. What is the compression factor for this gas at the prevailing temperature and pressure?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found...
Questions


Mathematics, 05.03.2021 22:20


Mathematics, 05.03.2021 22:20

Mathematics, 05.03.2021 22:20


Mathematics, 05.03.2021 22:20



Mathematics, 05.03.2021 22:20





Mathematics, 05.03.2021 22:20

English, 05.03.2021 22:20

English, 05.03.2021 22:20

Mathematics, 05.03.2021 22:20

Mathematics, 05.03.2021 22:20

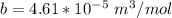
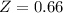
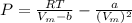
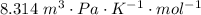 for R , 273K for T ,
for R , 273K for T , 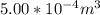 for
for 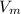 ,
, 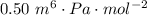 for a and
for a and 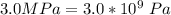 for P
for P