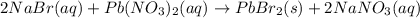The correct chemical equation for the reaction of sodium bromide and lead nitrate is: .
a. NaBr(aq) + Pb(NO3)2aq) >PbBr2ts) + NaNO3(aq)
b. NaBr(aq) + Pb(NO3)2> PbBr2(aq) + NaNO3(s)
c. 2 NaBr(aq) + Pb(NO3)2(aq)> PbBr2(aq) + NaNO3(s)
d. 2 NaBr(aq) + Pb(NO3)2(aq)> PbBr2 + NaNO3(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
The correct chemical equation for the reaction of sodium bromide and lead nitrate is: .
a. NaBr(aq)...
Questions




English, 06.12.2021 14:00

Chemistry, 06.12.2021 14:00



English, 06.12.2021 14:00

History, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00

Spanish, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00

Spanish, 06.12.2021 14:00


English, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00