
Chemistry, 09.09.2020 23:01 j1theking18
What are the final hydrogen ion concentration and pH of a solution obtained by mixing 400mL of 0.2M NaOH with 150mL of 0.1M H3PO4?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 09:00
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
What are the final hydrogen ion concentration and pH of a solution obtained by mixing 400mL of 0.2M...
Questions

History, 25.02.2021 17:20



Mathematics, 25.02.2021 17:20


Mathematics, 25.02.2021 17:20




Mathematics, 25.02.2021 17:20

Mathematics, 25.02.2021 17:20




Chemistry, 25.02.2021 17:20





Mathematics, 25.02.2021 17:20

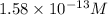
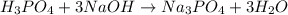
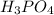 reacts with 3 mole NaOH.
reacts with 3 mole NaOH.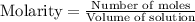
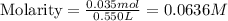
![[OH^-]](/tpl/images/0748/0545/b2910.png) = 0.0636 M
= 0.0636 M![pOH=-\log [OH^-]](/tpl/images/0748/0545/1fac1.png)
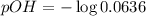
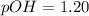
![pH=-\log [H^+]](/tpl/images/0748/0545/37e81.png)
![12.8=-\log [H^+]](/tpl/images/0748/0545/12b90.png)
![[H^+]=1.58\times 10^{-13}M](/tpl/images/0748/0545/66dc4.png)


