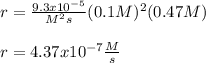The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M and [O2] = 3.7 M, the observed rate of the reaction is 0.00022022 M/s. (a) What is the value of the rate constant? (d) What is the rate of reaction when [NO] = 0.1 M and [O2] = 0.47 M?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1
You know the right answer?
The reaction 2NO(g) + O2(g) 2NO2(g) is second order in NO and first order in O2. When [NO] = 0.8 M a...
Questions


History, 23.03.2021 04:50

History, 23.03.2021 04:50

Mathematics, 23.03.2021 04:50


Geography, 23.03.2021 04:50



Mathematics, 23.03.2021 04:50







Computers and Technology, 23.03.2021 04:50




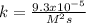
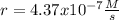
![r=k[NO]^2[O_2]](/tpl/images/0747/3983/b9fab.png)
![k=\frac{r}{[NO]^2[O_2]}\\\\k=\frac{0.00022022M/s}{(0.8M)^2(3.7M)} \\\\k=\frac{9.3x10^{-5}}{M^2s}](/tpl/images/0747/3983/c4bb6.png)