
Chemistry, 08.09.2020 14:01 awesomegrill
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201 decays to a mercury isotope. Express your answer as a nuclear equation. Part CGold-198 undergoes beta emission. Express your answer as a nuclear equation. Part DGold-188 decays by positron emission. Express your answer as a nuclear equation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201...
Questions





Mathematics, 11.11.2020 06:10


Mathematics, 11.11.2020 06:10

World Languages, 11.11.2020 06:10




Mathematics, 11.11.2020 06:10

Biology, 11.11.2020 06:10

Advanced Placement (AP), 11.11.2020 06:10

Mathematics, 11.11.2020 06:10

Computers and Technology, 11.11.2020 06:10

Social Studies, 11.11.2020 06:10



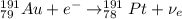
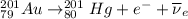
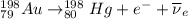
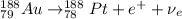
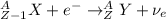
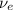 ) is emitted.
) is emitted. 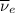 ) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant). 


