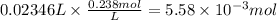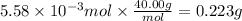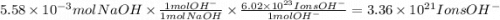Chemistry, 08.09.2020 14:01 amandaestevez030
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissolved in 23.46 mL?
b. How many individual hydroxide ions (OH) are found in 23.46 mL?
c. How many moles of sulfuric acid, H2SO4, are neutralized by 23.46 mL of 0.238 M NaOH(aq)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissol...
Questions





Biology, 04.08.2019 18:30

Mathematics, 04.08.2019 18:30

English, 04.08.2019 18:30


History, 04.08.2019 18:30


Mathematics, 04.08.2019 18:30

History, 04.08.2019 18:30

Mathematics, 04.08.2019 18:30


Mathematics, 04.08.2019 18:30


Geography, 04.08.2019 18:30



History, 04.08.2019 18:30