
Chemistry, 06.09.2020 02:01 anthonybowie99
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The volume of a sphere with a radius r is V = (4/3)πr3; the density of gold is 19.3 g/cm^3.)
b. A cube of platinum of edge length 0.021 mm (density = 21.4 g/cm3).
c. 37.3 mL of ethanol (density = 0.798 g/mL).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The vol...
Questions




Computers and Technology, 07.05.2020 02:07


History, 07.05.2020 02:07

Mathematics, 07.05.2020 02:07

English, 07.05.2020 02:07




Mathematics, 07.05.2020 02:07








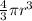
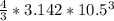 = 4849.68 cm^3
= 4849.68 cm^3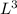 =
= 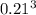 = 9.26 x 10^9 cm^3
= 9.26 x 10^9 cm^3


