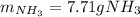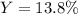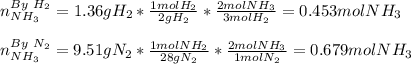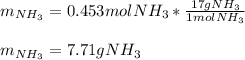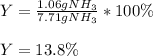Chemistry, 05.09.2020 22:01 Lindsay882
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is the theoretical yield in grams for this reaction under the given conditions? 2.)What is the percent yield for this reaction under the given conditions?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2

Chemistry, 23.06.2019 16:00
Express your answer using two significant figures. 1.7 km^2
Answers: 1

Chemistry, 23.06.2019 16:30
Ascientist measures the vapor pressure of a liquid. at 15° c, the vapor pressure was 19 kilopascals. at 25° c, it was 31 kilopascals. at 35° c, it was 59 kilopascals. which statement can be inferred from the observations?
Answers: 3
You know the right answer?
3H2(g)+N2(g)→2NH3(g) 1.36 g H2 is allowed to react with 9.51 g N2, producing 1.06 g NH3 1.) What is...
Questions


Mathematics, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10



Physics, 04.05.2021 21:10

History, 04.05.2021 21:10

History, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10


Mathematics, 04.05.2021 21:10



Advanced Placement (AP), 04.05.2021 21:10

English, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10

Mathematics, 04.05.2021 21:10