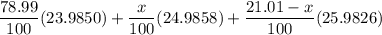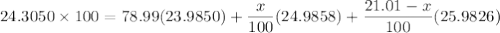Chemistry, 05.09.2020 01:01 starlightmoon213
There are three stable isotopes of magnesium. Their masses are 23.9850, 24.9858, and 25.9826 u. If the average atomic mass of magnesium is 24.3050 u and the natural abundance of the lightest isotope is 78.99%, what are the natural abundances of the other two isotopes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
There are three stable isotopes of magnesium. Their masses are 23.9850, 24.9858, and 25.9826 u. If t...
Questions


Chemistry, 30.08.2020 02:01

World Languages, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01


Mathematics, 30.08.2020 02:01

Biology, 30.08.2020 02:01


Mathematics, 30.08.2020 02:01

English, 30.08.2020 02:01

Arts, 30.08.2020 02:01

Social Studies, 30.08.2020 02:01



Mathematics, 30.08.2020 02:01

History, 30.08.2020 02:01

Biology, 30.08.2020 02:01


English, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01