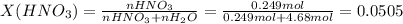Chemistry, 04.09.2020 23:01 0465834wu1
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid. 2.56×10−2 0.102 5.33×10−2 5.11×10−2 The density of the solution is needed to solve the problem.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

You know the right answer?
Calculate the mole fraction of nitric acid of a(n) 15.7% (by mass) aqueous solution of nitric acid....
Questions

Mathematics, 03.12.2020 20:10

Social Studies, 03.12.2020 20:10




Computers and Technology, 03.12.2020 20:10




Mathematics, 03.12.2020 20:10




Mathematics, 03.12.2020 20:10

Biology, 03.12.2020 20:10

History, 03.12.2020 20:10

Mathematics, 03.12.2020 20:10

Mathematics, 03.12.2020 20:10

Mathematics, 03.12.2020 20:10

Mathematics, 03.12.2020 20:10