
Chemistry, 03.09.2020 21:01 ilovecatsomuchlolol
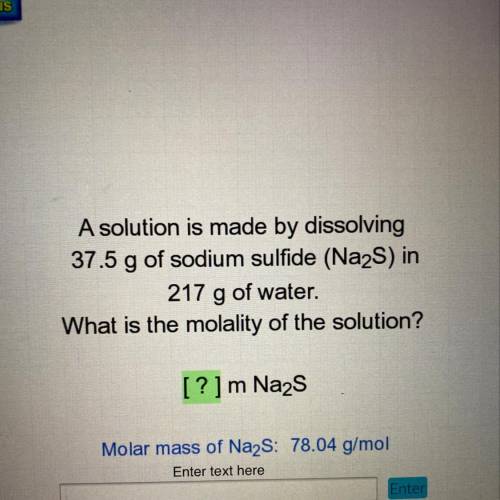
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
What is the molality of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
Wha...
217 g of water.
Wha...
Questions




Mathematics, 27.06.2019 01:00


Biology, 27.06.2019 01:00


Arts, 27.06.2019 01:00

Mathematics, 27.06.2019 01:00




Biology, 27.06.2019 01:00


Mathematics, 27.06.2019 01:00

History, 27.06.2019 01:00




Mathematics, 27.06.2019 01:00



