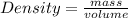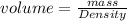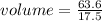Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 18:30
Sediment that settles on the bottom of the sea may form layers of rock which description tells how this sediment is deposited. a.)randomely b.)vertically c.)diagonally d.)horizontally
Answers: 2

Chemistry, 24.06.2019 00:40
Ionic solids such as sodium chloride are easily shattered, but metallic solids such as copper can be easily bent and shaped. this difference occurs because a. ionic solids have low melting points b. atoms in metallic solids are not arranged in a regular pattern c. covalent bonding between sodium and chlorine keeps the solid rigid d. mobile electrons in the copper can shift without disrupting the solid
Answers: 1
You know the right answer?
The density of a substance is 17.5 g/mL. Determine the volume (in mL) of 63.6 g of this substance....
Questions



Mathematics, 06.03.2020 19:19


Mathematics, 06.03.2020 19:19



History, 06.03.2020 19:19






Mathematics, 06.03.2020 19:19


Mathematics, 06.03.2020 19:19

Mathematics, 06.03.2020 19:19

Mathematics, 06.03.2020 19:19

Spanish, 06.03.2020 19:19