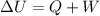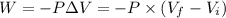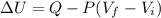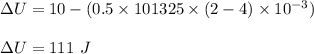A gas absorbs 10J of heat and is simultaneously
compressed by a const and external pressure
of...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Questions


Geography, 17.08.2019 16:10

Geography, 17.08.2019 16:10


Geography, 17.08.2019 16:10



Computers and Technology, 17.08.2019 16:10

History, 17.08.2019 16:10











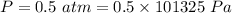
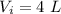 .
.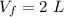 .
.