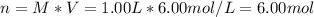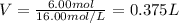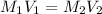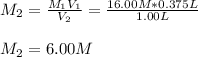Chemistry, 02.09.2020 04:01 matt199296
It is desired to make 1.00 liter of 6.00 M nitric acid from concentrated 16.00 M HNO3.A) How many moles of nitric acid are in 1.00 L of 6.00 M nitric acid?B) What volume of concentrated 16.00 M nitric acid will contain this number of moles?C) If this volume of concentrated nitric acid (answer to b) is diluted to 1.00 liter, what will be the molarity of the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
It is desired to make 1.00 liter of 6.00 M nitric acid from concentrated 16.00 M HNO3.A) How many mo...
Questions



History, 12.10.2021 03:40


Biology, 12.10.2021 03:40

Biology, 12.10.2021 03:40



Physics, 12.10.2021 03:40


Chemistry, 12.10.2021 03:40

English, 12.10.2021 03:40

Engineering, 12.10.2021 03:40


History, 12.10.2021 03:40

Mathematics, 12.10.2021 03:40

Mathematics, 12.10.2021 03:40


Mathematics, 12.10.2021 03:40

Chemistry, 12.10.2021 03:50