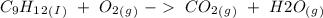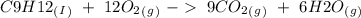Chemistry, 01.09.2020 21:01 heids17043
You may want to reference (Page) Section 12.4 while completing this problem. Enter the balanced chemical equation for the complete combustion of each of the following hydrocarbons found in gasoline used to start a fire:.
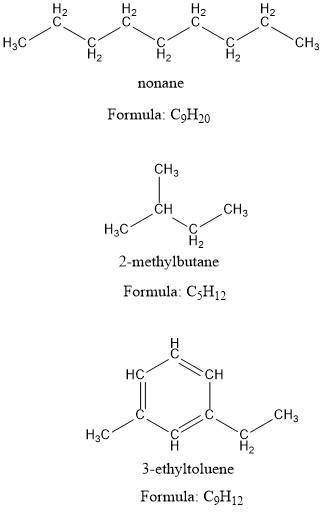
Part A. liquid nonane
Express your answer as a chemical equation including phases.
Part B. liquid isopentane (2-methylbutane)
Express your answer as a chemical equation including phases.
Part C. liquid 3-ethyltoluene
Express your answer as a chemical equation including phases.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

You know the right answer?
You may want to reference (Page) Section 12.4 while completing this problem. Enter the balanced chem...
Questions


Mathematics, 10.02.2021 02:20

History, 10.02.2021 02:20




Arts, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20


Social Studies, 10.02.2021 02:20

Computers and Technology, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20


Mathematics, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20


Mathematics, 10.02.2021 02:20

Mathematics, 10.02.2021 02:20

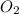 ) and the products are Carbon dioxide (
) and the products are Carbon dioxide (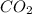 ) and Water (
) and Water (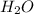 ). Additionally, for the states, we will have (l) for the liquid state and (g) for the gas state. So, we can analyze each reaction:
). Additionally, for the states, we will have (l) for the liquid state and (g) for the gas state. So, we can analyze each reaction: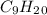 , with this in mind we can write the combustion reaction:
, with this in mind we can write the combustion reaction: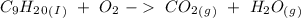
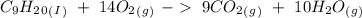
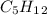 , with this in mind we can write the combustion reaction:
, with this in mind we can write the combustion reaction: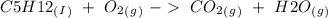
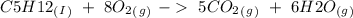
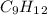 , with this in mind we can write the combustion reaction:
, with this in mind we can write the combustion reaction: