
Chemistry, 31.08.2020 23:01 eskarletche8
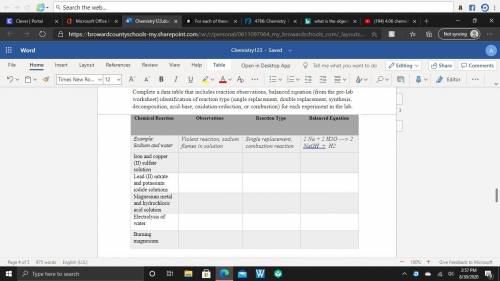
Data: Complete a data table that includes reaction observations, balanced equation (from the pre-lab worksheet) identification of reaction type (single replacement, double replacement, synthesis, decomposition, acid-base, oxidation-reduction, or combustion) for each experiment in the lab. Lead (II) nitrate and potassium iodide solutions Magnesium metal and hydrochloric acid solution Electrolysis of water Burning magnesium

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
Data: Complete a data table that includes reaction observations, balanced equation (from the pre-lab...
Questions


Mathematics, 22.07.2020 22:01


Mathematics, 22.07.2020 22:01

Mathematics, 22.07.2020 22:01


Mathematics, 22.07.2020 22:01



English, 22.07.2020 22:01





Mathematics, 22.07.2020 22:01

Mathematics, 22.07.2020 22:01

Mathematics, 22.07.2020 22:01



Physics, 22.07.2020 22:01



