
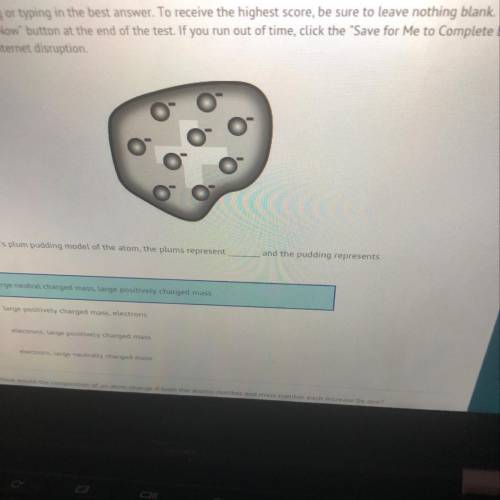
In J. J. Thomson's plum pudding model of the atom, the plums represent
and the pudding represents
A
large neutral charged mass, large positively charged mass
B)
large positively charged mass, electrons
electrons, large positively charged mass
electrons, large neutrally charged mass

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
In J. J. Thomson's plum pudding model of the atom, the plums represent
and the pudding represents
Questions



Geography, 26.07.2019 14:30


Biology, 26.07.2019 14:30


Biology, 26.07.2019 14:30

Mathematics, 26.07.2019 14:30


Biology, 26.07.2019 14:30


Biology, 26.07.2019 14:30


Mathematics, 26.07.2019 14:30


Biology, 26.07.2019 14:30


World Languages, 26.07.2019 14:30


History, 26.07.2019 14:30



