
Chemistry, 31.08.2020 02:01 sarrivera579
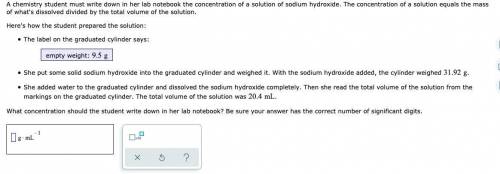
A chemistry student must write down in her lab notebook the concentration of a solution of sodium hydroxide. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: The label on the graduated cylinder says: empty weight: She put some solid sodium hydroxide into the graduated cylinder and weighed it. With the sodium hydroxide added, the cylinder weighed . She added water to the graduated cylinder and dissolved the sodium hydroxide completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was . What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1


Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
A chemistry student must write down in her lab notebook the concentration of a solution of sodium hy...
Questions

Computers and Technology, 20.02.2021 01:30

Mathematics, 20.02.2021 01:30


Social Studies, 20.02.2021 01:30


English, 20.02.2021 01:30


Geography, 20.02.2021 01:30

Mathematics, 20.02.2021 01:30

Mathematics, 20.02.2021 01:30

Mathematics, 20.02.2021 01:30


English, 20.02.2021 01:30

English, 20.02.2021 01:30


Biology, 20.02.2021 01:30

History, 20.02.2021 01:30



Physics, 20.02.2021 01:30



