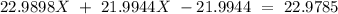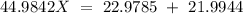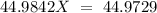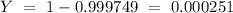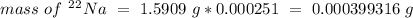Chemistry, 31.08.2020 01:01 Blackhawk1881
Sodium only has one naturally occuring isotope, Na23 , with a relative atomic mass of 22.9898 u. A synthetic, radioactive isotope of sodium, Na22 , is used in positron emission tomography. Na22 has a relative atomic mass of 21.9944 u. A 1.5909 g sample of sodium containing a mixture of Na23 and Na22 has an apparent "atomic mass" of 22.9785 u . Find the mass of Na22 contained in this sample

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

You know the right answer?
Sodium only has one naturally occuring isotope, Na23 , with a relative atomic mass of 22.9898 u. A s...
Questions


Spanish, 02.04.2021 22:30


English, 02.04.2021 22:30







Mathematics, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30



Mathematics, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30

World Languages, 02.04.2021 22:30


Mathematics, 02.04.2021 22:30

Mathematics, 02.04.2021 22:30

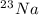 is an unknow value "X" and the molar fraction of
is an unknow value "X" and the molar fraction of 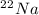 is "Y". We have to keep in mind that the molar fractions can be added:
is "Y". We have to keep in mind that the molar fractions can be added: