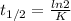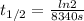Chemistry, 29.08.2020 01:01 kimbely7704
In a first-order reaction, the half-life is 139 minutes. What is the rate constant?
a. 8.31 x 10-5 s-1
b. 5770 s-1
c. 0.299 s-1
d. 4.99 x10-3s-1
e. 1.20 x 10-4s-1

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
In a first-order reaction, the half-life is 139 minutes. What is the rate constant?
a. 8.31 x 10-5...
Questions

English, 17.12.2020 19:40

Mathematics, 17.12.2020 19:40

Computers and Technology, 17.12.2020 19:40


Computers and Technology, 17.12.2020 19:40


Mathematics, 17.12.2020 19:40


Biology, 17.12.2020 19:40


Mathematics, 17.12.2020 19:40


Social Studies, 17.12.2020 19:40


Mathematics, 17.12.2020 19:40


Chemistry, 17.12.2020 19:40



Mathematics, 17.12.2020 19:40