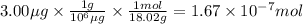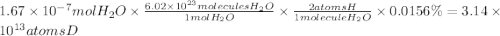For isotopic analysis, an ice core sample was heated to produce gaseous H2O. If 3.00 μg of gaseous H2O was injected into a mass spectrometer:
How many moles of water were injected? 1.67×10−7
If the sample contains 0.0156% deuterium, how many deuterium atoms were injected?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
For isotopic analysis, an ice core sample was heated to produce gaseous H2O. If 3.00 μg of gaseous H...
Questions


Mathematics, 16.10.2020 05:01


History, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Chemistry, 16.10.2020 05:01



Health, 16.10.2020 05:01



English, 16.10.2020 05:01



Physics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


Physics, 16.10.2020 05:01

History, 16.10.2020 05:01