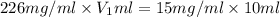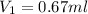Chemistry, 27.08.2020 23:01 joelpimentel
You have a stock solution that is 226mg/mL and you need 10mL of a working solution that is 15mg/mL. What is the volume of stock solution will you need to dilute to have 10mL of working solution at the above concentration. State your answer to two decimals.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

You know the right answer?
You have a stock solution that is 226mg/mL and you need 10mL of a working solution that is 15mg/mL....
Questions

History, 03.10.2019 02:40


Mathematics, 03.10.2019 02:40



Mathematics, 03.10.2019 02:40

Mathematics, 03.10.2019 02:40



Spanish, 03.10.2019 02:40

History, 03.10.2019 02:40

English, 03.10.2019 02:40

English, 03.10.2019 02:40

History, 03.10.2019 02:40




History, 03.10.2019 02:40

History, 03.10.2019 02:40

Biology, 03.10.2019 02:40

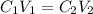
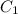 = concentration of stock solution = 226 mg/ml
= concentration of stock solution = 226 mg/ml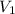 = volume of stock solution = ?
= volume of stock solution = ?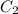 = concentration of working solution= 15 mg/ml
= concentration of working solution= 15 mg/ml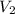 = volume of working solution= 10 ml
= volume of working solution= 10 ml