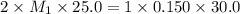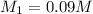Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
A 25.0 mL sample of sulfuric acid is titrated with 30.0 mL of 0.150 M sodium hydroxide. Calculate th...
Questions



Mathematics, 10.11.2021 18:50

Mathematics, 10.11.2021 18:50




Chemistry, 10.11.2021 18:50


Computers and Technology, 10.11.2021 18:50


Chemistry, 10.11.2021 19:00


History, 10.11.2021 19:00


Health, 10.11.2021 19:00



Mathematics, 10.11.2021 19:00

English, 10.11.2021 19:00

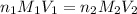
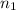 = basicity of sulphuric acid
= basicity of sulphuric acid 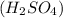 = 2
= 2 = Molarity of sulphuric acid
= Molarity of sulphuric acid 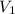 = volume of sulphuric acid
= volume of sulphuric acid  = acidity of sodium hydroxide
= acidity of sodium hydroxide 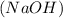 = 1
= 1 = Molarity of sodium hydroxide
= Molarity of sodium hydroxide 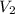 = volume of sodium hydroxide
= volume of sodium hydroxide