
Chemistry, 25.08.2020 01:01 HalpMahOnMahH0meW0rk
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate.
a)Calculate the volume of gas measured at r. t.p, produced in this reaction
b)calculatr the maximum mass of copper(ii)nitrate, produced in this reaction
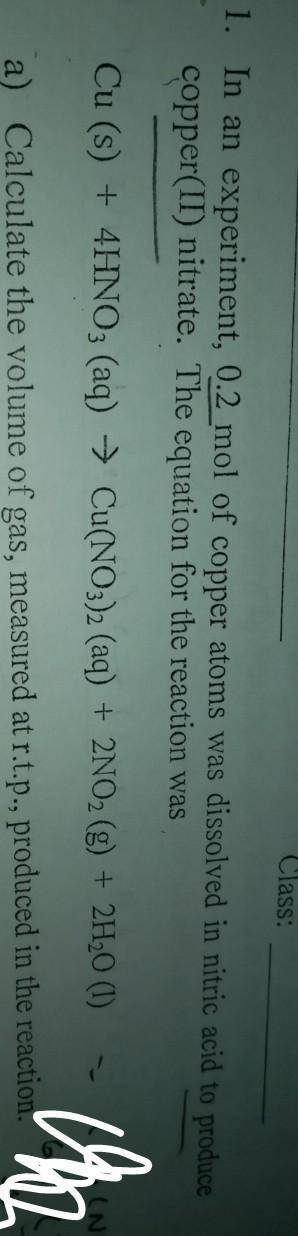

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate....
Questions

Biology, 28.10.2020 19:40


Mathematics, 28.10.2020 19:40

Computers and Technology, 28.10.2020 19:40


Mathematics, 28.10.2020 19:40


Spanish, 28.10.2020 19:40


Mathematics, 28.10.2020 19:40


English, 28.10.2020 19:40


Mathematics, 28.10.2020 19:40



Mathematics, 28.10.2020 19:40

Mathematics, 28.10.2020 19:40

Biology, 28.10.2020 19:40

Mathematics, 28.10.2020 19:40



