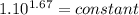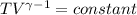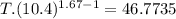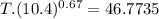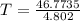Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 21:00
Using the thermodynamic information in the aleks data tab, calculate the boiling point of titanium tetrachloride ticl4. round your answer to the nearest degree.
Answers: 1
You know the right answer?
An ideal gas with γ = 1.67 has an initial temperature of 0°C, initial volume of 10.0 liters, and ini...
Questions

English, 17.09.2020 08:01

Chemistry, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Biology, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

English, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Chemistry, 17.09.2020 08:01

History, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Social Studies, 17.09.2020 08:01

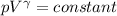 .
.