
Chemistry, 22.08.2020 19:01 stellaglenn205
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation:
2ClO2(aq) + 2OH−(aq) → ClO−2(aq) + ClO−3(aq) + H2O(l)
Under a certain set of conditions, the initial rate of disappearance of chlorine dioxide was determined to be 2.30 × 10−1 M/s. What is the initial rate of appearance of chlorite ion under those same conditions?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chem...
Questions

Geography, 08.10.2019 13:00

Mathematics, 08.10.2019 13:00

History, 08.10.2019 13:00

Mathematics, 08.10.2019 13:00


Mathematics, 08.10.2019 13:00

Geography, 08.10.2019 13:00

Chemistry, 08.10.2019 13:00

History, 08.10.2019 13:00


Mathematics, 08.10.2019 13:00

Mathematics, 08.10.2019 13:00

Physics, 08.10.2019 13:00

History, 08.10.2019 13:00


History, 08.10.2019 13:00



Mathematics, 08.10.2019 13:00

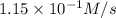
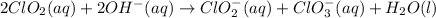
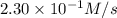
![\frac{d[ClO_2]}{2dt}=\frac{d[ClO_2^{-}]}{dt}](/tpl/images/0727/2886/91438.png)
![\frac{2.30\times 10^{-1}}{2}=\frac{d[ClO_2^{-}]}{dt}](/tpl/images/0727/2886/40dd2.png)
![\frac{d[ClO_2^{-}]}{dt}=1.15\times 10^{-1}M/s](/tpl/images/0727/2886/e1ee5.png)


