

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
Write the equilibrium constant expression for this reaction: 2H+(aq)+CO−23(aq) → H2CO3(aq)...
Questions





Physics, 28.01.2021 05:40








English, 28.01.2021 05:40

Mathematics, 28.01.2021 05:40

Mathematics, 28.01.2021 05:40



Mathematics, 28.01.2021 05:40

English, 28.01.2021 05:40

Mathematics, 28.01.2021 05:40

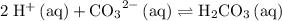 :
:![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \approx \frac{[\mathrm{H_2CO_3}]}{\left[\mathrm{H^{+}\, (aq)}\right]^{2} \, \left[\mathrm{CO_3}^{2-}\right]}](/tpl/images/0726/6258/84dbc.png) .
.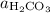 ,
, 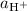 , and
, and 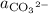 denote the activities of the three species, and
denote the activities of the three species, and ![[\mathrm{H_2CO_3}]](/tpl/images/0726/6258/0f634.png) ,
, ![\left[\mathrm{H^{+}}\right]](/tpl/images/0726/6258/81ca8.png) , and
, and ![\left[\mathrm{CO_3}^{2-}\right]](/tpl/images/0726/6258/1667f.png) denote the concentrations of the three species.
denote the concentrations of the three species.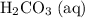 is the only product of this reaction. Besides, its coefficient in the balanced reaction is one. Therefore, the numerator would simply be
is the only product of this reaction. Besides, its coefficient in the balanced reaction is one. Therefore, the numerator would simply be 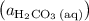 .
.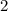 " on the product side of this reaction.
" on the product side of this reaction. 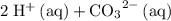 is equivalent to
is equivalent to 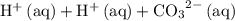 . The species
. The species 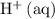 appeared twice among the reactants. Therefore, its activity should also appear twice in the denominator:
appeared twice among the reactants. Therefore, its activity should also appear twice in the denominator: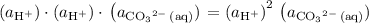 .
.![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \quad\begin{matrix}\leftarrow \text{from products} \\[0.5em] \leftarrow \text{from reactants}\end{matrix}](/tpl/images/0726/6258/9745b.png) .
.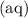 " species. Note that all the three species here are indeed aqueous. Hence, this equilibrium constant expression can be approximated as:
" species. Note that all the three species here are indeed aqueous. Hence, this equilibrium constant expression can be approximated as:![\displaystyle K = \frac{\left(a_{\mathrm{H_2CO_3\, (aq)}}\right)}{\left(a_{\mathrm{H^{+}}}\right)^2\, \left(a_{\mathrm{{CO_3}^{2-}\, (aq)}}\right)} \approx \frac{\left[\mathrm{H_2CO_3\, (aq)}\right]}{\left[\mathrm{H^{+}\, (aq)}\right]^2\cdot \left[\mathrm{{CO_3}^{2-}\, (aq)}\right]}](/tpl/images/0726/6258/9131e.png) .
.

