Chemistry, 22.08.2020 01:01 taysomoneyyy
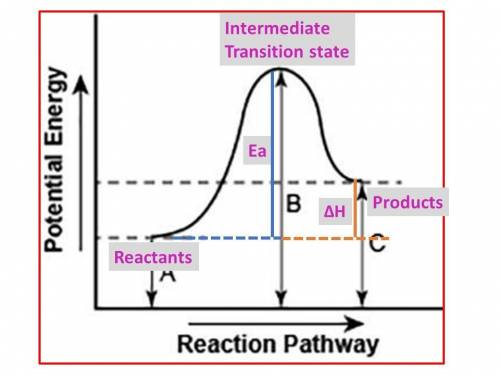
The diagram shows the potential energy changes for a reaction pathway. (10 points) A curve line graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The curve begins at a lower level and ends at a slightly higher level. A vertical line labeled A, starts from the x axis till the beginning of the graph line. A vertical line labeled B starts from the x axis and continues till the peak of the graph. Another vertical line labeled C is shown from the x axis till the point where the curve ends. Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative. Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points) A curve line grap...
Questions












Mathematics, 30.07.2021 03:00


Mathematics, 30.07.2021 03:00

Mathematics, 30.07.2021 03:00