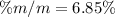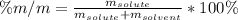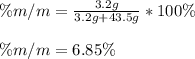Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

You know the right answer?
Calculate the mass percent of HOCH 2CH 2OH in a solution made by dissolving 3.2 g of HOCH 2CH 2OH in...
Questions


English, 10.09.2020 02:01


Social Studies, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

History, 10.09.2020 02:01


Physics, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Social Studies, 10.09.2020 02:01

Geography, 10.09.2020 02:01


Advanced Placement (AP), 10.09.2020 02:01

Arts, 10.09.2020 02:01

English, 10.09.2020 02:01

Chemistry, 10.09.2020 02:01

History, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

English, 10.09.2020 02:01